Vertebrate
| Vertebrate | |
|---|---|

| |
| Diversity of vertebrates: Acipenser oxyrinchus (Actinopterygii), an African bush elephant (Tetrapoda), a tiger shark (Chondrichthyes) and a river lamprey (Agnatha). | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Superphylum: | Deuterostomia |
| Phylum: | Chordata |
| Clade: | Olfactores |
| Subphylum: | Vertebrata J-B. Lamarck, 1801[2] |
| Infraphyla | |
| Synonyms | |
|
Ossea Batsch, 1788[2] | |
Vertebrates (/ˈvɜːrtəbrɪts, -ˌbreɪts/)[3] are animals with a backbone or spine, consisting of vertebrae and intervertebral discs. The vertebrae are irregular bones, and the intervertebral discs are of fibrocartilage. The vertebral column surrounds and protects the spinal cord. The other feature unique to vertebrates is the presence of a cranium, the bony dome of the skull.
The vertebrates make up the subphylum Vertebrata with some 65,000 species in the phylum Chordata. The vertebrates include mammals, birds, amphibians, and various classes of reptiles and fish. Classes of fish include the jawless (Agnatha), and the jawed (Gnathostomata). The jawed fish include both the cartilaginous fish and the bony fish. Bony fish include the lobe-finned fish, which gave rise to the tetrapods (four limbed vertebrates).
Vertebrates make up less than five percent of all described animal species; the rest are all invertebrates, that lack a backbone.
Etymology
[edit]The word vertebrate derives from the Latin word vertebratus, meaning jointed,[4] from vertebra meaning joint from Latin vertere to turn.[5]
Anatomy and morphology
[edit]All vertebrates are built along the basic chordate body plan of five synapomorphies. These are a rigid axial skeleton that includes a vertebral column developed around an elastic notochord. The notochord becomes the intervertebral discs, and runs dorsally to the gut tube along the length of an animal, hence the common name of backbone. [6] The axial endoskeleton typically continues beyond the anus/cloaca to form an elongated tail.[7] Some vertebrates evolved to become tailless with only a vestigial coccyx.[citation needed] A dorsal nerve cord, which folds and fuses into a hollow neural tube during embryonic development and eventually gives rise to the brain and spinal cord, runs more dorsally to the axial endoskeleton (enclosed by protective skeletal extensions known as neural arches), with a fore-end enlargement that is contained within a distinct skeletonized braincase (hence the alternative name for vertebrates, the craniates).[citation needed] All vertebrate embryos develop transient pharyngeal arches, which in fish develop into the branchial arches that support the gills. Other vertebrate features are a jaw, hyoid and/or the middle ear ossicles.[citation needed] An iodine-concentrating organ called the endostyle, which functions as a filter feeding organ in aquatic animals has evolved into the thyroid in most vertebrates.[citation needed]
Vertebrates vary in body length ranging from the frog species Brachycephalus pulex, a Brazilian flea toad, with a minimum adult snout–vent length of 6.45 millimetres (0.254 in)[8] to the blue whale, at up to 33 m (108 ft).
Vertebral column
[edit]
With only one exception, the defining characteristic of all vertebrates is the vertebral column, in which the embryonic notochord found in all chordates is replaced by a segmented series of mineralized elements called vertebrae separated by fibrocartilaginous intervertebral discs, which are embryonic and evolutionary remnants of the notochord. Hagfish are the only extant vertebrate whose notochord persists and is not integrated/ replaced by the vertebral column. A few vertebrates have secondarily lost this feature and retain the notochord into adulthood, such as the sturgeon.[9]
Gills
[edit]
Most vertebrates are aquatic and carry out gas exchange via gills. The gills are carried right behind the head, bordering the posterior margins of a series of crescentic openings from the pharynx to the outside. Each gill is supported by a cartilaginous or bony gill arch,[10] which develop embryonically from pharyngeal arches. Bony fish have three pairs of gill arches, cartilaginous fish have five to seven pairs, while the primitive jawless fish have seven pairs. The ancestral vertebrates likely had more arches than seven, as some of their chordate relatives have more than 50 pairs of gill opens,[7] although most, if not all, of these openings are actually involved in filter feeding rather than respiration. In jawed vertebrates, the first gill arch pair evolved into the jointed jaws and form an additional oral cavity ahead of the pharynx. Research also suggests that the sixth branchial arch contributed to the formation of the vertebrate shoulder, which separated the head as a distinct part of the body.[11]
In amphibians and some primitive bony fishes, the larvae bear external gills, branching off from the gill arches.[12] These are reduced in adulthood, their respiratory function taken over by the internal gills proper in fishes and by cutaneous respiration in most amphibians. While some amphibians such as axolotl retain the external gills into adulthood, the complex internal gill system as seen in fish apparently being irrevocably lost very early in the evolution of tetrapods, who evolved lungs (which are homologous to swim bladders) to breathe air.[13]
While the more specialized terrestrial vertebrates lack gills, the gill arches form during fetal development, and form the basis of essential structures such as jaws, the thyroid gland, the larynx, the columella (corresponding to the stapes in mammals) and, in mammals, the malleus and incus.[7]
Central nervous system
[edit]The central nervous system of vertebrates is based on the embryonic dorsal nerve cord (which then flattens into a neural plate before folding and fusing over into a hollow neural tube) running along the dorsal aspect of the notochord. Of particular importance and unique to vertebrates is the presence of neural crest cells, which are progenitor cells critical to coordinating the functions of cellular components.[14] Neural crest cells migrate through the body from the dorsal nerve cord during development, initiate the formation of neuronal ganglia and various special sense organs.[15][16][17] The peripheral nervous system forms when neural crest cells branch out laterally from the dorsal nerve cord and migrate together with the mesodermal somites to innervate the various different structures that develop in the body.[citation needed]
The vertebrates are the only chordate group with neural cephalization, and their neural functions are centralized towards a series of enlarged clusters in the head, which give rise to a brain. A slight swelling of the anterior end of the nerve cord is found in invertebrate chordates such as lancelets (a sister subphylum known as the cephalochordates), though it lacks eyes and other complex special sense organs comparable to those of vertebrates. Other chordates do not show any trends towards cephalization.[7]
The rostral end of the neural tube is expanded by a thickening of the walls and expansion of the central canal of spinal cord into three primary brain vesicles: the prosencephalon (forebrain), mesencephalon (midbrain) and rhombencephalon (hindbrain), which are further differentiated in the various vertebrate groups.[18] Two laterally placed retinas and optical nerves form around outgrowths from the midbrain, except in hagfish which may have secondarily lost the structures.[19][20] The forebrain is more well-developed in most tetrapods and subdivided into the telencephalon and diencephalon, while the midbrain dominates in fish and some salamanders. In vertebrates with paired appendages, especially tetrapods, a pair of secondary enlargements of the hindbrain become the cerebella, which modulate complex motor coordinations. The brain vesicles are usually bilaterally symmetrical, giving rise to the paired cerebral hemispheres in mammals.[18]
The resultant anatomy of a central nervous system arising from a single nerve cord dorsal to the gut tube, headed by a series of (typically paired) brain vesicles, is unique to vertebrates. This is in stark contrast to invertebrates with well-developed central nervous systems such as arthropods and cephalopods, which have an often ladder-like ventral nerve cord made of paired segmental ganglia on the opposite (ventral) side of the gut tube, with a split brain stem circumventing the foregut around each side to form a brain on the dorsal side of the mouth.[7] The higher functions of the vertebrate CNS are highly centralized towards the brain (particularly the forebrain), while the invertebrate CNS is significantly more decentralized with the segmental ganglia having substantial neural autonomy independent of the brain (which itself is a fused cluster of segmental ganglia from the rostral metameres).[citation needed]
Molecular signatures
[edit]Molecular markers known as conserved signature indels (CSIs) in protein sequences have been identified and provide distinguishing criteria for the subphylum Vertebrata.[21] Specifically, 5 CSIs in the following proteins: protein synthesis elongation factor-2 (EF-2), eukaryotic translation initiation factor 3 (eIF3), adenosine kinase (AdK) and a protein related to ubiquitin carboxyl-terminal hydrolase are exclusively shared by all vertebrates and reliably distinguish them from all other metazoan.[21] A specific relationship between vertebrates and tunicates is strongly supported by two CSIs found in the proteins Rrp44 (associated with exosome complex) and serine palmitoyltransferase, that are exclusively shared by species from these two subphyla but not cephalochordates, indicating vertebrates are more closely related to tunicates than cephalochordates.[21]
Evolutionary history
[edit]External relationships
[edit]Originally, the "Notochordata hypothesis" suggested that the Cephalochordata is the sister taxon to Craniata (Vertebrata). This group, called the Notochordata, was placed as sister group to the Tunicata (Urochordata). Although this was once the leading hypothesis,[22] studies since 2006 analyzing large sequencing datasets strongly support Olfactores (tunicates + vertebrates) as a monophyletic clade,[23][24][21] and the placement of Cephalochordata as sister-group to Olfactores (known as the "Olfactores hypothesis"). As chordates, they all share the presence of a notochord, at least during a stage of their life cycle.[citation needed]
The following cladogram summarizes the systematic relationships between the Olfactores (vertebrates and tunicates) and the Cephalochordata.
| Chordata |
| ||||||||||||
First vertebrates
[edit]
Vertebrates originated during the Cambrian explosion, which saw a rise in organism diversity. The earliest known vertebrates belongs to the Chengjiang biota[25] and lived about 518 million years ago.[1] These include Haikouichthys, Myllokunmingia,[25] Zhongjianichthys,[26] and probably Haikouella.[27] Unlike the other fauna that dominated the Cambrian, these groups had the basic vertebrate body plan: a notochord, rudimentary vertebrae, and a well-defined head and tail.[28] All of these early vertebrates lacked jaws in the common sense and relied on filter feeding close to the seabed.[29][page needed] A vertebrate group of uncertain phylogeny, small eel-like conodonts, are known from microfossils of their paired tooth segments from the late Cambrian to the end of the Triassic.[30]
From fish to amphibians
[edit]
The first jawed vertebrates may have appeared in the late Ordovician (~445 mya) and became common in the Devonian period, often known as the "Age of Fishes".[31] The two groups of bony fishes, the Actinopterygii and Sarcopterygii, evolved and became common.[32] The Devonian also saw the demise of virtually all jawless fishes save for lampreys and hagfishes, as well as the Placodermi, a group of armoured fish that dominated the entirety of that period since the late Silurian as well as the eurypterids, dominant animals of the preceding Silurian, and the anomalocarids. By the middle of the Devonian, several droughts, anoxic events and oceanic competition led a lineage of sarcopterygii to leave water, eventually establishing themselves as terrestrial tetrapods in the succeeding Carboniferous.[citation needed]
Mesozoic
[edit]Amniotes branched from amphibious tetrapods early in the Carboniferous period. The synapsid amniotes were dominant during the late Paleozoic, the Permian, while diapsid amniotes became dominant during the Mesozoic. In the sea, the teleosts and sharks became dominant. Mesothermic synapsids called cynodonts gave rise to endothermic mammals and diapsids called dinosaurs eventually gave rise to endothermic birds, both in the Jurassic.[33]
After the Mesozoic
[edit]The Cenozoic world saw great diversification of bony fishes, amphibians, reptiles, birds and mammals.[34][35]
Over half of all living vertebrate species (about 32,000 species) are fish (non-tetrapod craniates), a diverse set of lineages that inhabit all the world's aquatic ecosystems, from the Tibetan stone loach (Triplophysa stolickai) in western Tibetan hot springs near Longmu Lake at an elevation of 5,200 metres (17,100 feet) to an unknown species of snailfish (genus Pseudoliparis) in the Izu–Ogasawara Trench at a depth of 8,336 metres (27,349 feet).[36][37] Many fish varieties are the main predators in most of the world's freshwater and marine water bodies . The rest of the vertebrate species are tetrapods, a single lineage that includes amphibians (with roughly 7,000 species); mammals (with approximately 5,500 species); and reptiles and birds (with about 20,000 species divided evenly between the two classes). Tetrapods comprise the dominant megafauna of most terrestrial environments and also include many partially or fully aquatic groups (e.g., sea snakes, penguins, cetaceans).[citation needed]
Classification
[edit]There are several ways of classifying animals. Evolutionary systematics relies on anatomy, physiology and evolutionary history, which is determined through similarities in anatomy and, if possible, the genetics of organisms. Phylogenetic classification is based solely on phylogeny.[38] Evolutionary systematics gives an overview; phylogenetic systematics gives detail. The two systems are thus complementary rather than opposed.[39]
Traditional classification
[edit]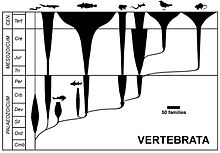
Conventional classification has extant vertebrates grouped into seven classes based on traditional interpretations of gross anatomical and physiological traits. The commonly held classification lists three classes of fish and four of tetrapods.[40]
- Subphylum Vertebrata
- Class Agnatha (jawless fishes)
- Class Chondrichthyes (cartilaginous fishes)
- Class Osteichthyes (bony fishes)
- Class Amphibia (amphibians)
- Class Reptilia (reptiles)
- Class Aves (birds)
- Class Mammalia (mammals)
In addition to these, there are two classes of extinct armoured fishes, the Placodermi and the Acanthodii, both considered paraphyletic.
Other ways of classifying the vertebrates have been devised, particularly with emphasis on the phylogeny of early amphibians and reptiles. An example based on Janvier (1981, 1997), Shu et al. (2003), and Benton (2004)[41] is given here († = extinct):

- Subphylum Vertebrata
- †Palaeospondylus
- Infraphylum Agnatha or Cephalaspidomorphi (lampreys and other jawless fishes)
- Superclass †Anaspidomorphi (anaspids and relatives)
- Infraphylum Gnathostomata (vertebrates with jaws)
- Class †Placodermi (extinct armoured fishes)
- Class Chondrichthyes (cartilaginous fishes)
- Class †Acanthodii (extinct spiny "sharks")
- Superclass Osteichthyes (bony fishes)
- Class Actinopterygii (ray-finned bony fishes)
- Class Sarcopterygii (lobe-finned fishes, including the tetrapods)
- Superclass Tetrapoda (four-limbed vertebrates)
- Class Amphibia (amphibians, some ancestral to the amniotes)—now a paraphyletic group
- Class Synapsida (mammals and the extinct mammal-like reptiles)
- Class Sauropsida (reptiles and birds)
While this traditional classification is orderly, most of the groups are paraphyletic, i.e. do not contain all descendants of the class's common ancestor.[41] For instance, descendants of the first reptiles include modern reptiles, mammals and birds; the agnathans have given rise to the jawed vertebrates; the bony fishes have given rise to the land vertebrates; the traditional "amphibians" have given rise to the reptiles (traditionally including the synapsids or mammal-like "reptiles"), which in turn have given rise to the mammals and birds. Most scientists working with vertebrates use a classification based purely on phylogeny,[42] organized by their known evolutionary history and sometimes disregarding the conventional interpretations of their anatomy and physiology.
Phylogenetic relationships
[edit]In phylogenetic taxonomy, the relationships between animals are not typically divided into ranks but illustrated as a nested "family tree" known as a phylogenetic tree. The cladogram below is based on studies compiled by Philippe Janvier and others for the Tree of Life Web Project and Delsuc et al.,[43][44] and complemented (based on,[45][46] and [47]). A dagger (†) denotes an extinct clade, whereas all other clades have living descendants.
| Vertebrata/ |
|
†"Ostracodermi" †"Placodermi" | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Craniata |
As shown in this cladogram, the †"Ostracodermi" (armoured jawless fishes) and †"Placodermi" (armoured jawed fishes) are paraphylectic groups, separated from gnathostomes and eugnathostomes respectively.[48][49]
Teleostei (Neopterygii) and Tetrapoda (amphibians, mammals, reptiles, birds) each make up about 50% of today's vertebrate diversity, while all other groups are either extinct or rare. The next cladogram shows the extant clades of tetrapods (the four-limbed vertebrates), and a selection of extinct (†) groups:
The placement of hagfish on the vertebrate tree of life has been controversial. Their lack of proper vertebrae (among with other characteristics of lampreys and jawed vertebrates) led phylogenetic analyses based on morphology to place them outside Vertebrata.[50] Molecular data, however, indicates they are vertebrates closely related to lampreys.[51][52] An older view is that they are a sister group of vertebrates in the common taxon of Craniata.[53] A study by Miyashita et al. (2019), reconciled the two types of analysis, supporting the Cyclostomata hypothesis using only morphological data.[54]
| |||||||
Number of extant species
[edit]The number of described vertebrate species are split between tetrapods and fish. The following table lists the number of described extant species for each vertebrate class as estimated in the IUCN Red List of Threatened Species, 2014.3.[55]
| Vertebrate groups | Image | Class | Estimated number of described species[55][56] |
Group totals[55] | ||
|---|---|---|---|---|---|---|
| Anamniote lack amniotic membrane so need to reproduce in water |
Jawless | Fish | Myxini (hagfish) |
78 | >32,900 | |
| Hyperoartia (lamprey) |
40 | |||||
| Jawed | cartilaginous fish |
>1,100 | ||||
| ray-finned fish |
>32,000 | |||||
| lobe-finned fish |
8 | |||||
| Tetrapods | amphibians | 7,302 | 33,278 | |||
| Amniote have amniotic membrane adapted to reproducing on land |
reptiles | 10,711 | ||||
| mammals | 5,513 | |||||
| birds | 10,425 | |||||
| Total described species | 66,178 | |||||
The IUCN estimates that 1,305,075 extant invertebrate species have been described,[55] which means that less than 5% of the described animal species in the world are vertebrates.
Species databases
[edit]The following databases maintain (more or less) up-to-date lists of vertebrate species:
- Fish: Fishbase
- Amphibians: Amphibiaweb
- Reptiles: Reptile Database
- Birds: Avibase
- Mammals: Mammal species of the World
Population trends
[edit]The Living Planet Index, following 16,704 populations of 4,005 species of vertebrates, shows a decline of 60% between 1970 and 2014.[57] Since 1970, freshwater species declined 83%, and tropical populations in South and Central America declined 89%.[58] The authors note that, "An average trend in population change is not an average of total numbers of animals lost."[58] According to WWF, this could lead to a sixth major extinction event.[59] The five main causes of biodiversity loss are land-use change, overexploitation of natural resources, climate change, pollution and invasive species.[60]
See also
[edit]- Marine vertebrate – Marine animals with a vertebrate column
- Invertebrate
- Endoskeleton
- Skeletal system of the horse
- Taxonomy of the vertebrates (Young, 1962) – Classification of spine-possessing animals according to some authorities
References
[edit]- ^ a b Yang, Chuan; Li, Xian-Hua; Zhu, Maoyan; Condon, Daniel J.; Chen, Junyuan (2018). "Geochronological constraint on the Cambrian Chengjiang biota, South China" (PDF). Journal of the Geological Society. 175 (4): 659–666. Bibcode:2018JGSoc.175..659Y. doi:10.1144/jgs2017-103. S2CID 135091168. Archived (PDF) from the original on 9 October 2022.
- ^ a b Nielsen, C. (July 2012). "The authorship of higher chordate taxa". Zoologica Scripta. 41 (4): 435–436. doi:10.1111/j.1463-6409.2012.00536.x. S2CID 83266247.
- ^ "vertebrate". Dictionary.com Unabridged (Online). n.d.
- ^ "vertebrate". Online Etymology Dictionary. Dictionary.com.
- ^ "Definition of Vertebra". www.merriam-webster.com. 25 November 2024. Retrieved 26 November 2024.
- ^ Waggoner, Ben. "Vertebrates: More on Morphology". UCMP. Retrieved 13 July 2011.
- ^ a b c d e Romer, A.S. (1949): The Vertebrate Body. W.B. Saunders, Philadelphia. (2nd ed. 1955; 3rd ed. 1962; 4th ed. 1970)
- ^ Bolaños, Wendy H.; Dias, Iuri Ribeiro; Solé, Mirco (7 February 2024). "Zooming in on amphibians: Which is the smallest vertebrate in the world?". Zoologica Scripta. 53 (4): 414–418. doi:10.1111/zsc.12654. S2CID 267599475.
- ^ Liem, K. F.; Walker, W. F. (2001). Functional anatomy of the vertebrates: an evolutionary perspective. Harcourt College Publishers. p. 277. ISBN 978-0-03-022369-3.
- ^ Scott, T. (1996). Concise encyclopedia biology. Walter de Gruyter. p. 542. ISBN 978-3-11-010661-9.
- ^ Brazeau, Martin D.; Castiello, Marco; El Fassi El Fehri, Amin; Hamilton, Louis; Ivanov, Alexander O.; Johanson, Zerina; Friedman, Matt (20 November 2023). "Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle". Nature. 623 (7987): 550–554. Bibcode:2023Natur.623..550B. doi:10.1038/s41586-023-06702-4. hdl:10044/1/107350. PMC 10651482. PMID 37914937.
- ^ Szarski, Henryk (1957). "The Origin of the Larva and Metamorphosis in Amphibia". The American Naturalist. 91 (860): 283–301. doi:10.1086/281990. JSTOR 2458911. S2CID 85231736.
- ^ Clack, J. A. (2002): Gaining ground: the origin and evolution of tetrapods. Indiana University Press, Bloomington, Indiana. 369 pp
- ^ Teng, Lu; Labosky, Patricia A. (2006). "Neural Crest Stem Cells". Neural Crest Induction and Differentiation. Advances in Experimental Medicine and Biology. Vol. 589. pp. 206–212. doi:10.1007/978-0-387-46954-6_13. ISBN 978-0-387-35136-0. PMID 17076284.
- ^ Gans, C.; Northcutt, R. G. (1983). "Neural crest and the origin of vertebrates: a new head". Science. 220 (4594): 268–273. Bibcode:1983Sci...220..268G. doi:10.1126/science.220.4594.268. PMID 17732898. S2CID 39290007.
- ^ Bronner, M. E.; LeDouarin, N. M. (1 June 2012). "Evolution and development of the neural crest: An overview". Developmental Biology. 366 (1): 2–9. doi:10.1016/j.ydbio.2011.12.042. PMC 3351559. PMID 22230617.
- ^ Dupin, E.; Creuzet, S.; Le Douarin, N.M. (2007) "The Contribution of the Neural Crest to the Vertebrate Body". In: Jean-Pierre Saint-Jeannet, Neural Crest Induction and Differentiation, pp. 96–119, Springer Science & Business Media. ISBN 9780387469546. doi:10.1007/978-0-387-46954-6_6. Full text
- ^ a b Hildebrand, M.; Gonslow, G. (2001): Analysis of Vertebrate Structure. 5th edition. John Wiley & Sons, Inc. New York
- ^ "Keeping an eye on evolution". PhysOrg.com. 3 December 2007. Retrieved 4 December 2007.
- ^ "Hyperotreti". tolweb.org.
- ^ a b c d Gupta, Radhey S. (January 2016). "Molecular signatures that are distinctive characteristics of the vertebrates and chordates and supporting a grouping of vertebrates with the tunicates". Molecular Phylogenetics and Evolution. 94 (Pt A): 383–391. Bibcode:2016MolPE..94..383G. doi:10.1016/j.ympev.2015.09.019. PMID 26419477.
- ^ Stach, Thomas (2008). "Chordate phylogeny and evolution: a not so simple three-taxon problem". Journal of Zoology. 276 (2): 117–141. doi:10.1111/j.1469-7998.2008.00497.x.
- ^ Delsuc, F (2006). "Tunicates and not cephalochordates are the closest living relatives of vertebrates" (PDF). Nature. 439 (7079): 965–968. Bibcode:2006Natur.439..965D. doi:10.1038/nature04336. PMID 16495997. S2CID 4382758. Archived (PDF) from the original on 9 October 2022.
- ^ Dunn, C.W. (2008). "Broad phylogenetic sampling improves resolution of the animal tree of life". Nature. 452 (7188): 745–749. Bibcode:2008Natur.452..745D. doi:10.1038/nature06614. PMID 18322464. S2CID 4397099.
- ^ a b Shu, D-G.; Luo, H-L.; Conway Morris, S.; Zhang, X-L.; Hu, S-X.; Chen, L.; Han, J.; Zhu, M.; Li, Y.; Chen, L-Z. (1999). "Lower Cambrian vertebrates from south China". Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965. S2CID 4402854.
- ^ Shu, D. (2003). "A paleontological perspective of vertebrate origin". Chinese Science Bulletin. 48 (8): 725–735. doi:10.1360/03wd0026.
- ^ Chen, J.-Y.; Huang, D.-Y.; Li, C.-W. (1999). "An early Cambrian craniate-like chordate". Nature. 402 (6761): 518–522. Bibcode:1999Natur.402..518C. doi:10.1038/990080. S2CID 24895681.
- ^ Waggoner, B. "Vertebrates: Fossil Record". UCMP. Archived from the original on 29 June 2011. Retrieved 15 July 2011.
- ^ Tim Haines, T.; Chambers, P. (2005). The Complete Guide to Prehistoric Life. Firefly Books.
- ^ Donoghue, P. C. J.; Forey, P. L.; Aldridge, R. J. (May 2000). "Conodont affinity and chordate phylogeny". Biological Reviews. 75 (2): 191–251. doi:10.1111/j.1469-185X.1999.tb00045.x. PMID 10881388. S2CID 22803015.
- ^ Encyclopædia Britannica: a new survey of universal knowledge, Volume 17. Encyclopædia Britannica. 1954. p. 107.
- ^ Berg, L. R.; Solomon, E. P.; Martin, D. W. (2004). Biology. Cengage Learning. p. 599. ISBN 978-0-534-49276-2.
- ^ Cloudsley-Thompson, J. L. (2005). Ecology and behaviour of Mesozoic reptiles. Springer. p. 6. ISBN 9783540224211.
- ^ Pires, Mathias; Mankin, Brian; Silvestro, Daniele; Quental, Tiago (26 September 2018). "Diversification dynamics of mammalian clades during the K–Pg mass extinction". Biology Letters. 14 (9). doi:10.1098/rsbl.2018.0458. PMC 6170748. PMID 30258031.
- ^ Lowery, Christopher; Fraass, Andrew (8 April 2019). "Morphospace expansion paces taxonomic diversification after end Cretaceous mass extinction". Nature Ecology & Evolution. 3 (6): 900–904. Bibcode:2019NatEE...3..900L. doi:10.1038/s41559-019-0835-0. hdl:1983/fb08c3c1-c203-4780-bc90-5994ec1030ff. PMID 30962557. S2CID 102354122 – via Nature.
- ^ Kottelat, M. (2012). "Conspectus_cobitidum.pdf Conspectus cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei)" (PDF). The Raffles Bulletin of Zoology. Supplement No. 26: 1–199.
- ^ "Scientists find deepest fish ever recorded at 8,300 metres underwater near Japan". The Guardian. 3 April 2023. Retrieved 6 July 2024.
- ^ Andersen, N. M.; Weir, T. A. (2004). Australian water bugs: their biology and identification (Hemiptera-Heteroptera, Gerromorpha & Nepomorpha). Apollo Books. p. 38. ISBN 978-87-88757-78-1.
- ^ Hildebran, M.; Gonslow, G. (2001): Analysis of Vertebrate Structure. 5th edition. John Wiley & Sons, Inc. New York, page 33: Comment: The problem of naming sister groups
- ^ Campbell, Neil A. (1997). Biology. Buch (4th ed.). Menlo Park, Calif: Benjamin Cummings Publ. Comp. p. 632. ISBN 0805319409.
- ^ a b Benton, M.J. (1 November 2004). Vertebrate Palaeontology (Third ed.). Blackwell Publishing. pp. 33, 455 pp. ISBN 978-0632056378. Archived from the original on 19 October 2008. Retrieved 16 March 2006.
- ^ Irie, Naoki (26 December 2018). "The phylum Vertebrata: a case for zoological recognition". Zoological Letters. 4 Article Number 32: 32. doi:10.1186/s40851-018-0114-y. PMC 6307173. PMID 30607258.
- ^ Janvier, P. 1997. Vertebrata. Animals with backbones. Version 1 January 1997 (under construction). http://tolweb.org/Vertebrata/14829/1997.01.01 in The Tree of Life Web Project, http://tolweb.org/
- ^ Delsuc, F.; Philippe, H.; Tsagkogeorga, G.; Simion, P. (April 2018). "A phylogenomic framework and timescale for comparative studies of tunicates". BMC Biology. 16 (1). Tilak, M. K.; Turon, X.; López-Legentil, S.; Piette, J.; Lemaire, P.; Douzery, E. J.: 39. doi:10.1186/s12915-018-0499-2. PMC 5899321. PMID 29653534.
- ^ Friedman, Matt; Sallan, Lauren Cole (June 2012). "Five hundred million years of extinczion and recovery: A Phanerozoic survey of large-scale diversity patterns in fishes". Palaeontology. 55 (4): 707–742. Bibcode:2012Palgy..55..707F. doi:10.1111/j.1475-4983.2012.01165.x. S2CID 59423401.
- ^ Zhu, Min; Ahlberg, Per E.; Pan, Zhaohui; Zhu, Youan; Qiao, Tuo; et al. (21 October 2016). "A Silurian maxillate placoderm illuminates jaw evolution". Science. 354 (6310): 334–336. Bibcode:2016Sci...354..334Z. doi:10.1126/science.aah3764. PMID 27846567. S2CID 45922669.
- ^ Zhu, Min; Yu, Xiaobo; Ahlberg, Per Erik; Choo, Brian; Lu, Jing; et al. (25 September 2013). "A Silurian placoderm with osteichthyan-like marginal jaw bones". Nature. 502 (7470): 188–193. Bibcode:2013Natur.502..188Z. doi:10.1038/nature12617. PMID 24067611. S2CID 4462506.
- ^
 Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
Giles, Sam; Friedman, Matt; Brazeau, Martin D. (12 January 2015). "Osteichthyan-like cranial conditions in an Early Devonian stem gnathostome". Nature. 520 (7545): 82–85. Bibcode:2015Natur.520...82G. doi:10.1038/nature14065. ISSN 1476-4687. PMC 5536226. PMID 25581798.
- ^ Benton, Michael J. (2009). Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. p. 44. ISBN 978-1-4051-4449-0.
- ^ Ota, Kinya G.; Fujimoto, Satoko; Oisi, Yasuhiro; Kuratani, Shigeru (25 January 2017). "Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish". Nature Communications. 2: 373. Bibcode:2011NatCo...2..373O. doi:10.1038/ncomms1355. ISSN 2041-1723. PMC 3157150. PMID 21712821.
- ^ Kuraku; et al. (December 1999). "Monophyly of Lampreys and Hagfishes Supported by Nuclear DNA–Coded Genes". Journal of Molecular Evolution. 49 (6): 729–35. Bibcode:1999JMolE..49..729K. doi:10.1007/PL00006595. PMID 10594174. S2CID 5613153.
- ^ Stock, D.; Whitt, G. S. (7 August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfish form a natural group". Science. 257 (5071): 787–789. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- ^ Nicholls, H. (10 September 2009). "Mouth to Mouth". Nature. 461 (7261): 164–166. doi:10.1038/461164a. PMID 19741680.
- ^ Miyashita, Tetsuto; Coates, Michael I.; Farrar, Robert; Larson, Peter; Manning, Phillip L.; et al. (5 February 2019). "Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny". Proceedings of the National Academy of Sciences of the United States of America. 116 (6): 2146–2151. Bibcode:2019PNAS..116.2146M. doi:10.1073/pnas.1814794116. PMC 6369785. PMID 30670644.
- ^ a b c d The World Conservation Union. 2014. IUCN Red List of Threatened Species, 2014.3. Summary Statistics for Globally Threatened Species. Table 1: Numbers of threatened species by major groups of organisms (1996–2014).
- ^ Nelson, Joseph S. (2016). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-1-118-34233-6.
- ^ "Living Planet Report 2018". wwf.panda.org. Retrieved 21 May 2020.
- ^ a b Grooten, M.; Almond, R. E. A. (2018). Living Planet Report – 2018: Aiming Higher (PDF). WWF--World Wide Fund for Nature. ISBN 978-2-940529-90-2. Archived (PDF) from the original on 9 October 2022.
- ^ "WWF Finds Human Activity Is Decimating Wildlife Populations". Time. Retrieved 21 May 2020.
- ^ IPBES (25 November 2019). S. Diaz; J. Settele; E.S. Brondízio; et al. (eds.). Summary for policymakers of the global assessment report on biodiversity and ecosystem services. Bonn: IPBES Secretariat. pp. 1–56. doi:10.5281/zenodo.3553579.
Bibliography
[edit]- Kardong, Kenneth V. (1998). Vertebrates: Comparative Anatomy, Function, Evolution (second ed.). USA: McGraw-Hill. pp. 747 pp. ISBN 978-0-697-28654-3.
- "Vertebrata". Integrated Taxonomic Information System. Retrieved 6 August 2007.
External links
[edit]- Tree of Life
- Tunicates and not cephalochordates are the closest living relatives of vertebrates
- Vertebrate Pests chapter in United States Environmental Protection Agency and University of Florida/Institute of Food and Agricultural Sciences National Public Health Pesticide Applicator Training Manual
- The Vertebrates
- The Origin of Vertebrates Marc W. Kirschner, iBioSeminars, 2008.

